Centrifuge Hettich EBA 200
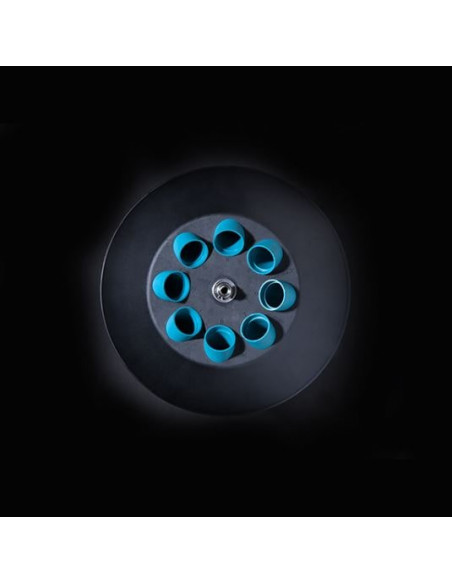
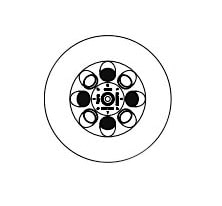
The Hettich EBA 200 is a compact clinical centrifuge designed for small sample volumes. It features a built-in, versatile 8-place fixed-angle rotor and an adjustable LCD display for convenient and quick operation. The centrifuge is equipped with a standard 8-place fixed-angle rotor that can accommodate standard blood and urine tubes with a capacity of up to 15 ml. The maximum capacity of the EBA 200 is 8 x 15 ml.

GPSR - General Product Safety Regulation
GPSR – General Product Safety Regulation
Responsible Economic Operator according to GPSR:
Andreas Hettich GmbH
Föhrenstraße 12
78532 Tuttlingen
Germany
Tel. +49 7461 7050
FAX: +49 7461 705 1125
https://www.hettichlab.com/de/kontakt/
Product Safety Sheet in Accordance with GPSR (General Product Safety Regulation) for the Hettich EBA 200 Centrifuge
1. Product Identification
- Product Name: Hettich EBA 200 Centrifuge
- Model Number: EBA 200 (including EBA 200 S)
- Manufacturer: Andreas Hettich GmbH & Co. KG
- WEEE-Reg. No.: DE 92954423
- Application: Medical device compliant with the IVD Directive 98/79/EC, specifically designed for human in-vitro diagnostics.
2. Intended Use
The Hettich EBA 200 centrifuge is designed for use in small laboratories, medical practices, veterinary clinics, and blood collection activities. It is used for the separation and preparation of substances, such as blood plasma, blood serum, and urine samples, via centrifugation.
3. Key Technical Data
- Max. Capacity: 8 x 15 ml
- Max. RCF: 3,461 | 6,153
- Max. Speed (RPM): 6,000 min⁻¹ | 8,000 min⁻¹
- Weight: 9 kg | 11 kg
- Cooling: Air cooling
- Dimensions (W x D x H): 261 x 353 x 228 mm
- Power Supply: Standard mains connection
4. Safety Information
-
General Safety Instructions:
- The device must only be operated by trained personnel.
- The device may only be used for its intended purpose.
- Read the user manual fully before first use.
- Sale is restricted to medical professionals in compliance with legal requirements.
-
Electrical Safety:
- Ensure the device is connected to a grounded outlet.
- Use only the power supply recommended by the manufacturer.
- Disconnect the power plug before cleaning or maintenance.
-
Mechanical Safety:
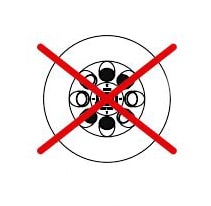
- The rotor must be loaded properly and symmetrically.
- Ensure the rotor is securely seated before operation.
- Never open the lid until the rotor has come to a complete stop.
- Do not exceed the maximum fill level specified for sample tubes.
-
Operational Safety:
- Avoid allowing liquids to enter the rotor chamber.
- Ensure that sample tubes are evenly filled to prevent vibrations and damage.
- Operate the centrifuge only in a clean and dry environment.
-
Overheating Protection:
- The device is equipped with overheating protection. It will automatically shut down if overheating occurs.
5. Health and Environmental Risks
-
Health Risks:
- Improper use may cause injuries due to unbalanced rotors or unsecured sample tubes.
- Contact with biological samples (e.g., blood serum) may pose health risks. Always wear appropriate protective equipment (e.g., gloves, safety goggles).
-
Environmental Risks:
- The device contains electronic components. Dispose of the device in accordance with WEEE regulations.
6. Emergency Measures
-
In Case of Malfunction:
- Immediately switch off the device and disconnect it from the mains.
- Inspect the rotor and rotor chamber for damage or foreign objects.
- Contact an authorized service provider.
-
In Case of Electrical Short Circuit:
- Disconnect the power supply (e.g., switch off the circuit breaker).
- Do not attempt to repair the device yourself.
7. Maintenance and Care
-
Cleaning:
- Clean the centrifuge with a soft cloth and mild detergent. Do not use harsh chemicals.
- Regularly remove dust and residues from the rotor and rotor chamber.
-
Maintenance:
- Have the device inspected at least once a year by the manufacturer or an authorized service provider.
- Replace wear parts, such as rotors and seals, as needed.
-
Storage:
- Store the device in a dry, dust-free environment.
8. Labels and Warnings
- Warning Symbols on the Device:
- ⚠️ Caution: Read the user manual.
- ⚡ Warning: Electrical hazard.
- ♻️ Dispose of in accordance with WEEE regulations.
9. User Responsibility
The user is responsible for:
- Adhering to the specified application areas.
- Ensuring regular maintenance and proper handling of the device.
- Training personnel in the use of the device.
- Complying with applicable regulations for medical devices.
10. Declaration of Conformity
The Hettich EBA 200 Centrifuge complies with the following:
- IVD Directive 98/79/EC
- WEEE Directive 2012/19/EU
- EMC Directive 2014/30/EU
- Low Voltage Directive 2014/35/EU
11. Contact Information
- Manufacturer: Andreas Hettich GmbH & Co. KG
- Address: Föhrenstr. 12, 78532 Tuttlingen, Germany
- Phone Number: +49 7461 705-0
- Email: info@hettichlab.com
- Website: www.hettichlab.com
12. Additional Notes
This product safety sheet has been prepared in accordance with the General Product Safety Regulation (GPSR). It does not replace the manufacturer’s user manual. The device must only be used by trained medical personnel.