PRP starter set | EBA 200 - Your solution for successful PRP production 🛍️🩸
A PRP starter set containing all the components required for PRP production
1x Hettich EBA 200 centrifuge
2x PRP tubes | Vi PRP-Pro PU 10 pcs.
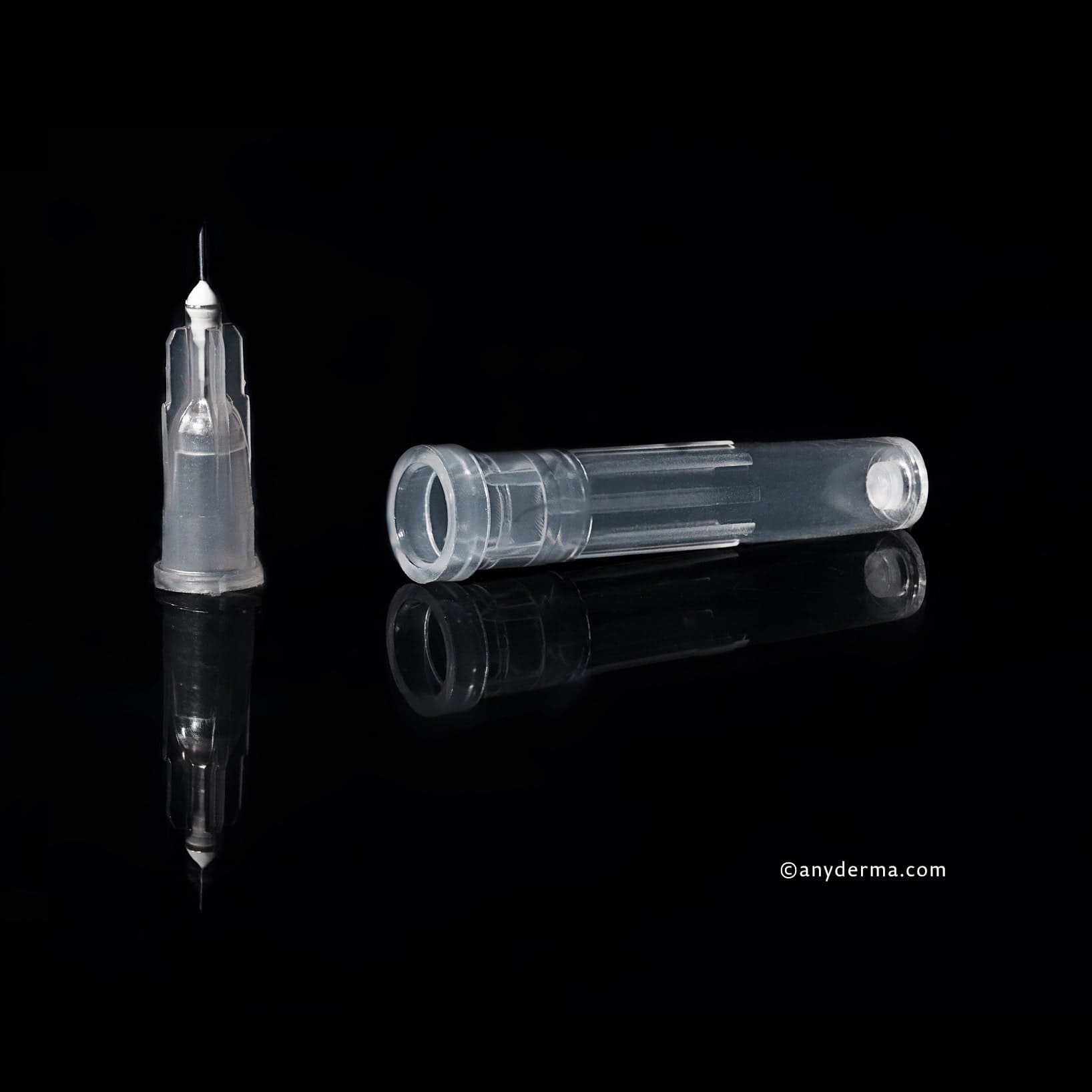
1x KIPIC® needle 25G x 42mm, PU 100 pcs.
1x KIPIC® Mesotherapy Needle 30G 4mm | PU 100 pcs.
1x KIPIC® Mesotherapy Needle 32Gx4mm | PU 100 pcs.
1x Mediware disposable syringes 5ml 3-piece Luer-Lock sterile (PU 100 pcs.)
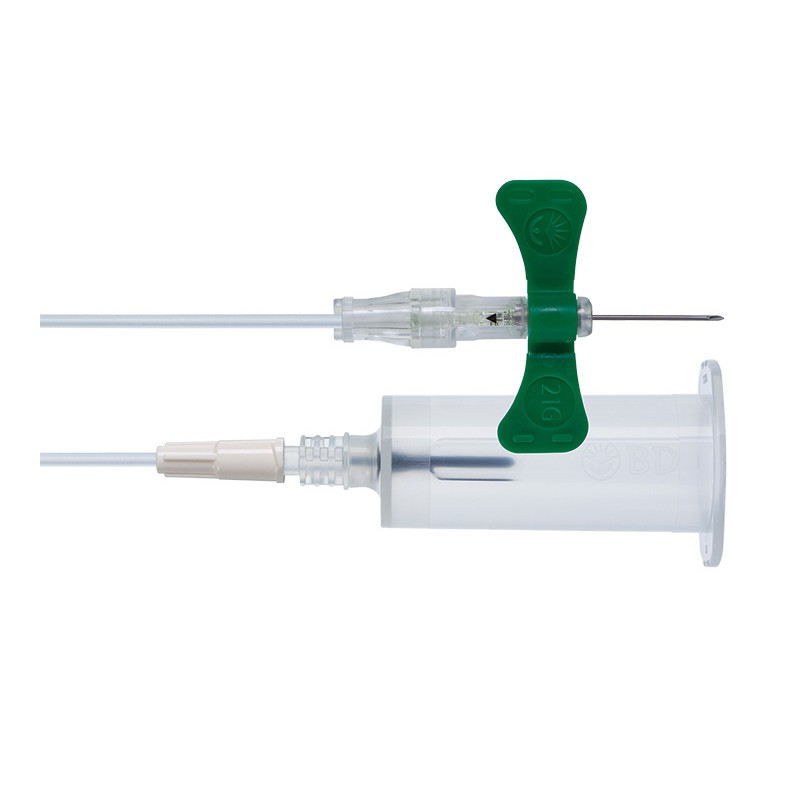
1x Safety Blood Collection Set + Luer Adapter 21G - PU 35 pcs.
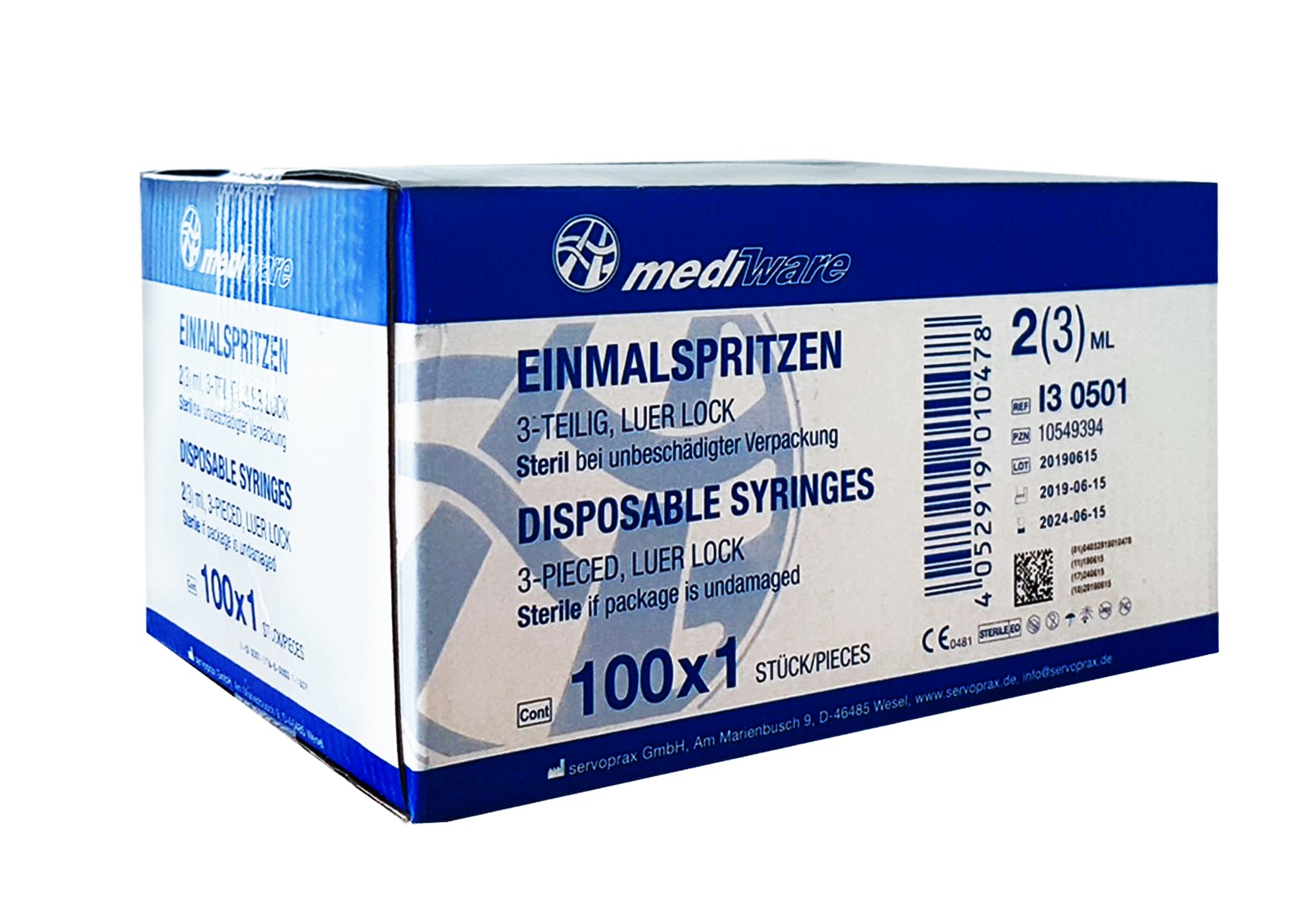
1x Mediware disposable syringes 2/3ml 3-piece Luer-Lock sterile (PU 100 pcs.)

- Features
GPSR – General Product Safety Regulation
Responsible Economic Operator according to GPSR:
prpmed Funkner e.K.
Kurstraße 7
63667 Nidda
Germany
Phone: +49 6043 9862 817
Email: kontakt@prpmed.de
Detailed GPSR Safety Regulations for the PRP Starter Set EBA 200
The PRP Starter Set EBA 200 is a medical device specifically designed for professional use in laboratory environments. It complies with the General Product Safety Regulation (GPSR), which governs product safety within the EU. To ensure the safety of users and patients, the following safety regulations must be strictly observed. These regulations cover the entire lifecycle of the device – from installation and operation to maintenance and disposal.
1. General Safety Requirements
-
Restricted Use by Qualified Personnel:
- This product is intended exclusively for use by trained and qualified professionals. Unauthorized or untrained individuals are not permitted to operate or maintain the device.
- The sale is restricted to professionals or their authorized purchasers in accordance with legal requirements for medical devices.
-
Compliance with Legal Standards:
- Users must adhere to national and European regulations for medical devices. In Germany, this includes the Medical Devices Act (MPG) and the Medical Device Operator Ordinance (MPBetreibV).
- Correct use is mandatory to minimize risks to both patients and operators.
-
Product Liability:
- The manufacturer is not liable for damages resulting from improper use, failure to follow safety instructions, or unauthorized modifications to the device.
-
Markings and Documentation:
- Ensure the device is marked with the CE certification, confirming compliance with all relevant EU directives.
- Keep the user manual and all related documentation readily available and follow all instructions.
2. Safety Regulations for Installation
-
Choosing the Installation Location:
- Place the EBA 200 on a stable, level, and vibration-free surface.
- The location must be free from direct sunlight, humidity, dust, and exposure to chemical fumes.
-
Electrical Safety:
- The device should only be connected to a grounded outlet that meets local electrical standards (e.g., DIN VDE standards in Germany).
- Do not use damaged cables, outlets, or extension cords. Replace any damaged cables immediately.
-
Environmental Conditions:
- The device is designed to operate in a temperature range of 10°C to 40°C.
- The relative humidity should be below 80%. Avoid exposing the device to extreme temperature fluctuations or condensation, as these can impair its performance.
3. Safety Regulations During Operation
-
Training and Instruction:
- Before first use, all personnel must undergo comprehensive training or instruction on how to operate the device.
- The user manual must be read and followed in its entirety.
-
Personal Protective Equipment (PPE):
- Always wear appropriate PPE, such as laboratory coats, protective goggles, and sterile gloves, to prevent contact with biological materials or hazardous substances.
-
Loading the Centrifuge:
- Ensure the centrifuge is symmetrically loaded to avoid imbalance during operation. Improper loading can cause vibrations, damage, or malfunctions.
- Only use approved tubes or containers that are compatible with the centrifuge.
-
Lid Safety:
- Before starting the device, the centrifuge lid must be fully closed and locked. Never operate the device if the lid is not properly secured.
-
Monitoring Operation:
- Do not leave the device unattended while it is running. If you notice unusual sounds, vibrations, or error messages, stop the device immediately and investigate the issue.
-
Emergency Stop Function:
- Familiarize yourself with the emergency stop function of the device to respond quickly in the event of a malfunction.
4. Maintenance and Cleaning
-
Regular Maintenance:
- Regular maintenance must be performed according to the manufacturer's guidelines. This includes inspections of:
- The lid seal and locking mechanism.
- The condition of mechanical and electrical components.
- Only qualified personnel should perform maintenance tasks.
- Regular maintenance must be performed according to the manufacturer's guidelines. This includes inspections of:
-
Cleaning:
- Clean the device after each use with appropriate medical-grade disinfectants approved for use with biological materials.
- Avoid harsh cleaning agents that may damage the device’s surface.
- Ensure no liquids enter the electronic components of the device.
-
Calibration:
- The device must be calibrated regularly to ensure precise performance. Calibration should only be done by authorized technicians or the manufacturer.
5. Safety Regulations for Disposal
-
Battery Disposal:
- If the device contains batteries or rechargeable cells, dispose of them in accordance with local waste management regulations for batteries. Use designated collection points.
-
Device Disposal:
- Medical devices, such as the PRP Starter Set EBA 200, are subject to specific disposal regulations. The device must not be disposed of with regular household waste.
- Contact the manufacturer or a certified waste disposal service provider to ensure proper disposal in compliance with legal requirements.
6. Emergency Procedures
-
Power Outage:
- In the event of a power outage during operation, immediately switch off the device and unplug it. Wait a few minutes after power is restored before restarting the device.
-
Accidents with Biological Materials:
- If biological samples are spilled or come into contact with surfaces, clean and disinfect the affected areas immediately. Always wear protective equipment while handling biological materials.
- Report any accidents according to your institution's internal protocols.
-
Technical Malfunctions:
- If the device malfunctions, such as emitting unusual noises or vibrations, switch it off immediately and unplug it. Contact the manufacturer or the customer service team for assistance.
7. Contact Information for Questions or Issues
For questions regarding operation, maintenance, or disposal of the PRP Starter Set EBA 200, please contact:
PRPMed
Kurstraße 7
63667 Nidda
Germany
Phone: +49 6043 9862 817
Email: kontakt@prpmed.de
Important Note: These safety regulations are developed in accordance with the General Product Safety Regulation (GPSR). Compliance with these regulations is mandatory to ensure the safety of both users and patients. For users outside the EU, please refer to the applicable regional safety regulations.